Super refining mineral, Zeolite's, chemical structure revealed
23 June 2008
For the first time, a European team of scientists has revealed the chemical structure of super use mineral, zeolite scolecite. This material forms around a third of the average packet of washing powder and helps refine 99 per cent of the world's petrol.
The material is also used to clean up nuclear waste.
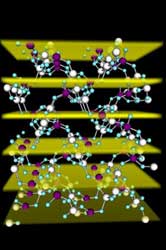 |
| The structure of zeolite scolecite, showing the aluminium and silicon atoms as the large sphere, connected by oxygen atoms in blue. The openings in the structure are the pores that serve to transport the molecules through the crystals to the active sites. The planes against which the scattering in the x-ray standing wave experiment occurs are highlighted; the experiment provides the distribution of aluminium and silicon within the crystal, yielding insights into where are the active sites in a zeolite. Photo: J. Van Bokhoven |
Zeolites are crystalline white minerals, mostly made of aluminium, silicon and oxygen. Their structure resembles molecular scaffolding, the same as a sieve. Because of this structure, they are frequently used as a ''molecular sieve,'' which means that with their pores can separate different molecules and cause different reactions. This is crucial in treating petrol and producing chemicals.
Zeolites can also provoke ion exchange, which is useful in water softening, or, in the removal of nuclear waste. This is achieved by filtering radioactive components.
The important, even critical, role they play in industry means that extensive research is carried out on the material around the world.
